When Doctors Adjust Doses After Switching to Generic Medications
Switching from a brand-name drug to a generic version seems simple-same active ingredient, lower cost, right? But for some medications, that switch isn’t as harmless as it looks. When doctors change doses after switching to generics, it’s not because they’re being cautious for no reason. It’s because certain drugs have narrow therapeutic index (NTI) ranges, where even tiny changes in blood levels can cause serious harm-or make the drug stop working entirely.
What Makes a Drug a Narrow Therapeutic Index (NTI) Drug?
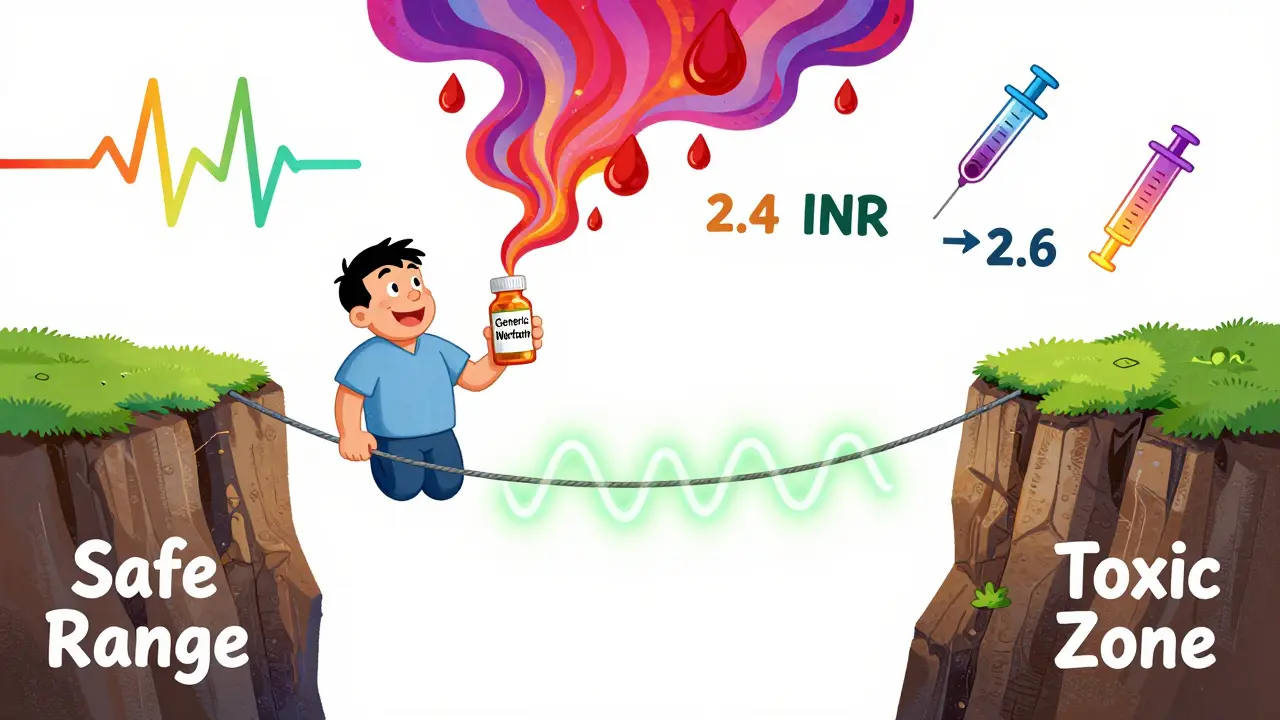
NTI drugs are the ones where the difference between a safe dose and a dangerous one is razor-thin. Think of it like walking a tightrope. A step too far one way, and you’re underdosed; a step too far the other, and you’re in toxic territory. These drugs don’t have room for error.
Examples include warfarin (a blood thinner), levothyroxine (for thyroid conditions), phenytoin and carbamazepine (for seizures), tacrolimus and cyclosporine (for transplant patients), and digoxin (for heart rhythm). For these, the target blood concentration often needs to stay within 20-30% of a specific value. If it dips below or rises above, bad things happen: seizures, blood clots, organ rejection, or heart rhythm problems.
The FDA defines NTI drugs as those where "small differences in dose or blood concentration may lead to serious therapeutic failures or serious adverse events." That’s not theoretical. It’s backed by data from thousands of real patients.
Why Do Generics Sometimes Cause Problems?
All generics must prove they’re "bioequivalent" to the brand name. That means, on average, they deliver the same amount of drug into the bloodstream within a range of 80-125% of the original. Sounds fine, right?
But here’s the catch: for NTI drugs, that 25% window is too wide. A patient stabilized on a brand-name drug at 5 mg might switch to a generic that delivers, on average, 10% more. That’s still within the legal range. But for someone on warfarin, a 10% increase could push their INR (a blood clotting measure) from 2.4 to 2.6-and suddenly, they’re at risk of bleeding.
Real-world studies show this isn’t just theory. One 2017 study found that patients switched between different generic versions of warfarin had a 23% higher chance of needing a dose adjustment within 30 days. For tacrolimus in transplant patients, 18.7% needed a dose change within two weeks of switching to a generic-compared to just 5.2% who stayed on the same product.
And it’s not just about the active ingredient. Fillers, coatings, and how the pill breaks down in the gut can vary between manufacturers. For a drug like levothyroxine, even minor differences in absorption can throw off thyroid hormone levels. Patients report fatigue, weight gain, or brain fog after a switch-even if their TSH levels are "in range." That’s because "in range" doesn’t always mean "feeling normal."
When Do Doctors Actually Change the Dose?
Most patients switching to generics don’t need a dose change. But for NTI drugs, doctors follow a simple rule: monitor before assuming.
Here’s what happens in practice:
- For warfarin: INR is checked within 7-14 days after the switch. If it’s more than 10% off the previous stable level, the dose is adjusted.
- For levothyroxine: TSH is retested at 6-8 weeks. If it’s outside the patient’s personal target range (not just the lab’s normal range), the dose is tweaked-usually in 12.5 mcg increments.
- For antiepileptics like phenytoin: Blood levels are drawn within two weeks. A change of more than 20% from the previous steady state triggers a dose review.
- For immunosuppressants like tacrolimus: Levels are checked every 3-7 days after a switch, especially in the first month after transplant.
These aren’t random checks. They’re based on guidelines from the American Epilepsy Society, the American College of Clinical Pharmacy, and hospital protocols like those at UF Health and Mayo Clinic.
Some patients never need a change. Others do-and they’re not "difficult" or "overreacting." They’re just physiologically sensitive. One patient might handle a generic switch with no issue. Another, with the same condition and same dose, might need a 15% increase. That’s why blanket rules don’t work.

Why Do Some Doctors Say It’s Not a Big Deal?
You’ll hear conflicting opinions. Some doctors say, "The FDA says generics are the same. Why overcomplicate it?" And technically, they’re right-for most drugs.
But here’s the reality: the FDA’s bioequivalence standards were designed for drugs like statins or antibiotics, where the window is wide. For NTI drugs, those standards were never meant to be the final word on clinical safety. The FDA itself acknowledges this. In its 2021 guidance, it says NTI drugs often require "therapeutic drug monitoring and small dose adjustments" because of their "steep exposure-response relationship."
Dr. Robert Temple of the FDA once wrote in JAMA that concerns are "often overstated." But even he didn’t say they were wrong. He said they’re rare. And for the patients they affect? Rare doesn’t matter. One seizure, one stroke, one rejected kidney-that’s 100% for them.
Meanwhile, academic pharmacists and hospital-based clinicians see the data daily. A 2022 survey of 1,247 hospital pharmacists found 68.3% had seen patients need dose adjustments after switching NTI generics. Antiepileptics, warfarin, and tacrolimus were the top three.
What Can You Do If You’re Switching?
If you’re on an NTI drug and your pharmacy switches your prescription to a generic:
- Ask your doctor: "Is this a drug that needs monitoring after a switch?" Don’t assume they know-many don’t.
- Request a follow-up test. Don’t wait for your next routine visit. Schedule it within the recommended window.
- Track your symptoms. Fatigue, mood changes, tremors, unusual bruising, or heart palpitations after a switch? Tell your doctor. Don’t wait for a lab result.
- Ask if you can stay on the same generic brand. If you’ve been stable on one, ask your doctor to write "Do Not Substitute" on the prescription. Some insurers will honor it.
- Know your numbers. Keep a log of your INR, TSH, or drug levels. Don’t rely on memory.
There’s no shame in asking for stability. For many patients, the goal isn’t just survival-it’s feeling normal again. That’s not a luxury. It’s part of treatment.
The Future: Tighter Standards, Better Generics
The FDA is moving toward tighter bioequivalence rules for NTI drugs. In 2023, they proposed a new standard: 90-111% instead of 80-125%. That’s a big deal. It means future generics will have to match the brand much more closely.
Some manufacturers are already ahead of the curve. Teva’s "TacroBell" tacrolimus, for example, shows 32% less variability between doses than standard generics. Specialty pharmacies are starting to stock these "supergenerics" for transplant and epilepsy patients.
But until those standards are fully in place, the responsibility falls on patients and doctors. The system isn’t broken-it’s just outdated for these high-risk drugs.
Switching to generics saves billions. That’s good. But saving money shouldn’t come at the cost of safety. For NTI drugs, the right approach isn’t to avoid generics-it’s to manage the switch wisely.
Stability matters. Monitoring matters. Communication matters. And sometimes, a 12.5 mcg change in levothyroxine isn’t a mistake. It’s the difference between feeling okay and feeling like you’re drowning in fatigue.
Do all generic drugs need dose adjustments after switching?
No. Only drugs with a narrow therapeutic index (NTI) typically require monitoring or dose changes after switching. These include warfarin, levothyroxine, phenytoin, tacrolimus, cyclosporine, and digoxin. For most other medications-like blood pressure pills, antibiotics, or antidepressants-switching to a generic is safe without any changes.
How long after switching to a generic should I get my blood tested?
It depends on the drug. For warfarin, test INR within 7-14 days. For levothyroxine, wait 6-8 weeks for TSH levels to stabilize. For antiepileptic drugs like phenytoin, check blood levels within 2 weeks. Always ask your doctor for the specific timeline based on your medication.
Can I ask my doctor to keep me on the same generic brand?
Yes. You can ask your doctor to write "Do Not Substitute" or "Brand Necessary" on your prescription. Some insurers will honor this, especially for NTI drugs. If your pharmacy switches anyway, you can appeal the decision or pay the difference out-of-pocket to stay on the version that works for you.
Why do some people have no issues after switching, while others do?
Everyone’s body absorbs and processes drugs differently. Factors like stomach acidity, gut bacteria, liver enzymes, and even diet can affect how a pill behaves. For NTI drugs, even small differences in absorption can push levels outside the safe range. One person might absorb a generic just as well as the brand. Another might not. It’s not about being "difficult"-it’s about biology.
Are newer generics safer than older ones?
Some are. Manufacturers like Teva and Aurobindo now produce "supergenerics" for NTI drugs with tighter quality controls and lower variability. These aren’t always labeled as such, but pharmacists can tell you which ones have better consistency. Ask if your pharmacy carries them. They’re often priced similarly to standard generics.
What to Do If You’re Still Unsure
If you’re on a high-risk medication and your doctor doesn’t mention monitoring after a switch, speak up. Bring this article. Print out the FDA’s NTI drug list. Ask: "Should I be tested after this switch?"
Don’t let cost savings override safety. The goal isn’t just to fill a prescription-it’s to keep you healthy. For NTI drugs, that means being proactive, not passive.
christy lianto
January 8, 2026 AT 20:57Wow. I had no idea switching generics could be this dangerous. I’m on levothyroxine and thought ‘same pill, cheaper’-turns out my brain fog wasn’t just stress. Got my TSH checked last week after a switch and it was off by 1.8. Doctor adjusted my dose and I feel like myself again. Don’t let anyone tell you it’s ‘all in your head.’
Ken Porter
January 9, 2026 AT 16:12Stop overcomplicating things. FDA says they’re equivalent. If your body can’t handle a generic, maybe you’re just weak. Get over it.
Molly Silvernale
January 10, 2026 AT 07:01Let’s be real-medicine isn’t math. It’s biology, it’s chaos, it’s the quiet trembling of a body trying to stay alive while the system cuts corners. A pill isn’t just a molecule-it’s a story. The same molecule, in a different coating, in a different gut, with a different liver, a different sleep cycle, a different cup of coffee at 7 a.m.-and suddenly, you’re not ‘in range,’ you’re in crisis. And who pays? The patient. Again. Always the patient. We’re not data points. We’re humans with hearts that skip, lungs that gasp, minds that fog… and someone’s spreadsheet says ‘close enough.’
It’s not about distrust. It’s about dignity. It’s about being allowed to feel like you’re not just surviving, but living. And if that means asking for the same generic brand, or a blood test, or a doctor who listens? Then that’s not being difficult. That’s being awake.
They call it ‘bioequivalence.’ I call it a gamble with your life. And I’m tired of being the one holding the dice.
swati Thounaojam
January 12, 2026 AT 03:19in india we dont even have choice, all generics only. i had seizure after switch, doctor said 'maybe you stress'... i cried. now i pay extra to get same brand. worth it.
Annette Robinson
January 13, 2026 AT 15:37If you’re on an NTI drug and you’ve switched generics, please, please, please get your labs checked. Don’t wait for symptoms to get bad. I’ve seen too many people suffer silently because they thought, ‘It’s just a generic, it should be fine.’ It’s not. Your body knows when something’s off-even if the numbers look ‘normal.’ Trust yourself. Advocate for yourself. You deserve to feel well, not just ‘not dead.’
Luke Crump
January 15, 2026 AT 09:32Oh wow, so now the FDA is the villain? And big pharma is the hero? Let me guess-next you’ll say insulin should cost $1000 a vial because ‘it’s complicated.’ This isn’t medicine. It’s fear-mongering dressed up as science. If generics were so dangerous, why are they used everywhere in the world? Why do we have 10x lower death rates in countries with 100% generic use? You’re not protecting patients-you’re protecting profits. Wake up.
Dave Old-Wolf
January 15, 2026 AT 13:07So if I’m on warfarin and they switch my generic, how soon do I get the INR test? Like, do I call the next day or wait a week? And what if my doctor says ‘no need’? Should I push harder?
Donny Airlangga
January 15, 2026 AT 18:37I’m a transplant nurse. I’ve watched patients crash after a generic switch-just because the pill looked different. Tacrolimus levels can drop like a stone. One guy went from 8.2 to 4.1 in 72 hours. He almost lost his kidney. We now have a protocol: no switch without a baseline and 3-day follow-up. It’s not paranoia. It’s practice. And if your doctor doesn’t know this? Educate them. Print this out. They’ll thank you later.
Kristina Felixita
January 17, 2026 AT 11:52My mom switched to a generic for her seizure med… and suddenly she couldn’t remember my name for 3 weeks. We thought it was dementia. Turned out her phenytoin level dropped 30%. We switched back to the original generic (same company, different batch) and boom-she was back. I’m telling everyone. If you’re on one of these meds, don’t let your pharmacy just swap it. Ask for the brand name. Ask for the maker. Write it down. It’s not crazy. It’s survival.