Stability Testing Requirements: Temperature and Time Conditions for Pharmaceutical Products

When a drug leaves the lab and enters the market, it needs to stay safe and effective for months-even years-under real-world conditions. That’s where stability testing comes in. It’s not optional. It’s not a suggestion. It’s a legal requirement enforced by global regulators like the FDA, EMA, and Health Canada. The core question is simple: How does this medicine hold up over time when exposed to heat, humidity, and light? The answers determine shelf life, storage rules, and ultimately, patient safety.
Why Temperature and Time Matter More Than You Think
Think about a bottle of pills sitting on your bathroom shelf. It’s warm. Humid. Maybe even exposed to sunlight. Now imagine that same bottle in a warehouse in Singapore or a delivery truck in Saudi Arabia. The conditions are wildly different. Stability testing simulates those extremes to catch problems before they reach patients. The science behind it is straightforward: heat and moisture speed up chemical breakdown. A drug might lose potency. It might form toxic byproducts. It might change shape-like a tablet crumbling or a liquid turning cloudy. These aren’t theoretical risks. In 2022, the FDA issued 27 warning letters specifically because companies failed to prove their products were stable. Some of those led to recalls. Patients got sick. Companies lost millions. That’s why the International Council for Harmonisation (ICH) set the global standard back in 2003 with ICH Q1A(R2). It’s still the rule today. No country has changed it. Not because it’s perfect-but because changing it globally is harder than building a new drug.The Three Testing Zones: Long-Term, Accelerated, and Intermediate
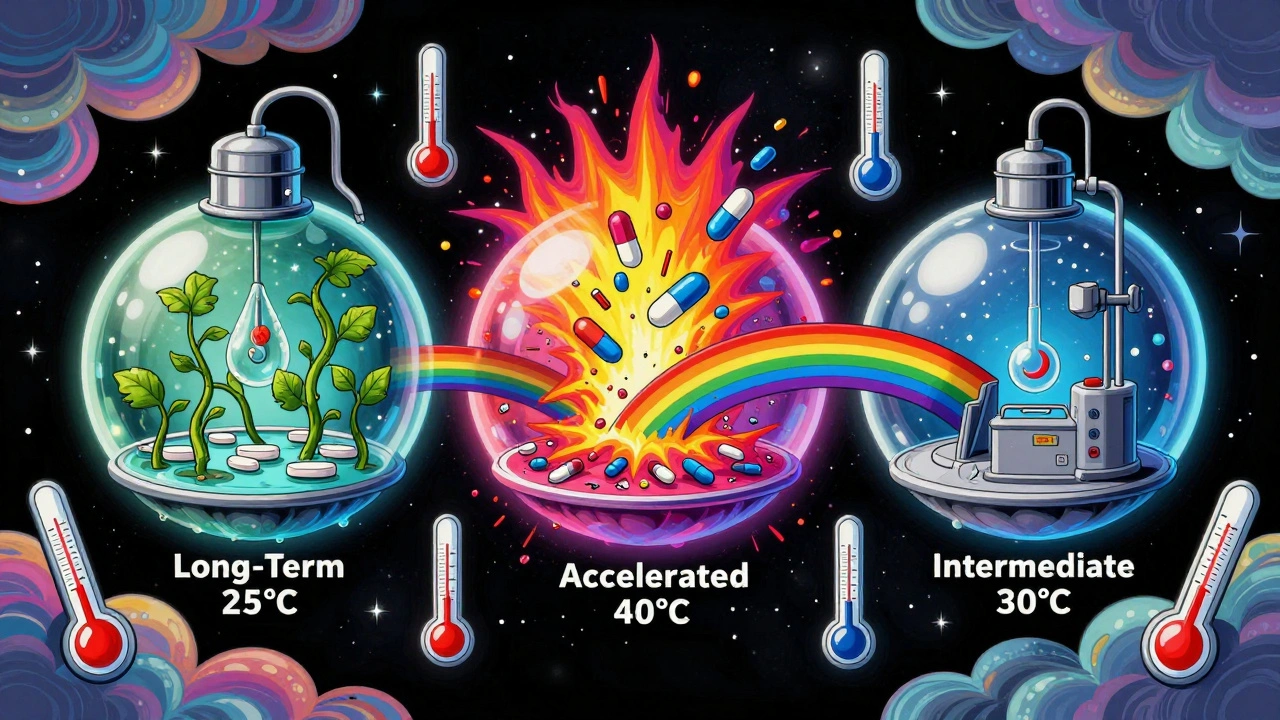
There are three main types of stability testing, each with fixed temperature and humidity settings. They’re not suggestions. They’re exact numbers you must hit-or your data gets rejected.- Long-term testing: This is the gold standard. It runs for years and tells you how long your drug will last under normal storage. Two options exist: 25°C ± 2°C at 60% RH ± 5% RH or 30°C ± 2°C at 65% RH ± 5% RH. You pick one based on where your product will be sold. If you’re targeting tropical markets like India or Brazil, you go with 30°C/65% RH. If you’re selling in Europe or North America, 25°C/60% RH is fine. The test must run at least 12 months before you can submit your application to regulators. The FDA insists on 12 months. The EMA lets you submit with 6 months if you’re planning to add the rest later.
- Accelerated testing: This is the stress test. You push the drug hard to see how fast it breaks down. The condition? 40°C ± 2°C at 75% RH ± 5% RH for exactly 6 months. This isn’t meant to mimic real life-it’s meant to predict it. If your drug fails here, you know you’ve got a problem. It’s the first red flag. If it passes, you can use the data to estimate shelf life. Studies show this 6-month test roughly predicts 24 months of real-time stability for 85% of small-molecule drugs. But it fails for hygroscopic drugs-those that soak up moisture like a sponge. Those need special handling.
- Intermediate testing: This is the backup plan. You only run it if your long-term test is at 25°C and your accelerated test shows a problem. The condition? 30°C ± 2°C at 65% RH ± 5% RH for 6 months. It’s not always required, but skipping it when you should’ve run it has cost companies recalls and delays.
What About Refrigerated or Frozen Drugs?
Not all medicines are kept at room temperature. Insulin, vaccines, and many biologics need to be cold. Their rules are different.- Refrigerated products: Long-term storage is 5°C ± 3°C for 12 months. Accelerated testing? Not at 40°C. That would destroy them. Instead, you test at 25°C ± 2°C at 60% RH ± 5% RH for 6 months. This simulates what happens if a fridge fails during shipping.
- Frozen products: These are the hardest to test. There’s no official ICH guideline yet. Most companies use -20°C or -70°C for long-term storage. Accelerated testing isn’t standardized. You’re on your own-unless you’re working with mRNA vaccines. Those need freeze-thaw cycle testing because each time they warm up and freeze again, they degrade. The standard ICH tests don’t catch that.
Global Climates Change the Rules
The world isn’t one climate. It’s five zones. ICH Q1A(R2) acknowledges that. If you’re selling globally, you need to test for all of them.- Zone I (Temperate): 21°C, 45% RH - Think Canada, Northern Europe.
- Zone II (Mediterranean/Subtropical): 25°C, 60% RH - Southern Europe, parts of the U.S.
- Zone III (Hot-Dry): 30°C, 35% RH - Middle East, parts of Australia.
- Zone IVa (Hot-Humid/Tropical): 30°C, 65% RH - Southeast Asia, India, Brazil.
- Zone IVb (Hot/Higher Humidity): 30°C, 75% RH - Coastal tropics, like parts of Indonesia.
How Often Do You Test? Timing Is Everything
Testing isn’t a one-time thing. You test at intervals: 0, 3, 6, 9, 12, 18, 24, and 36 months. The first few points are critical. That’s when degradation starts. If you only test at 12 and 24 months, you might miss a sudden drop in potency at 9 months. The chambers you use must be precise. Temperature must stay within ±0.5°C. Humidity within ±2% RH. That’s tighter than your home thermostat. If your chamber drifts to 26°C when it should be 25°C, your entire study could be invalidated. A 2023 LinkedIn survey of 142 stability professionals found that 78% had experienced at least one temperature excursion during a 12-month study. One slip-up. One failed batch. One delayed launch.
What Happens When You Get It Wrong?
Failures aren’t rare. They’re predictable. In 2021, Teva Pharmaceuticals had to recall 150,000 vials of Copaxone® because their stability tests didn’t catch protein aggregation at 40°C. The drug was safe-but less effective. Patients got worse. Merck, on the other hand, used intermediate testing at 30°C/65% RH to catch a polymorphic shift in Keytruda®. That’s the good kind of failure. They found it before it reached patients. They fixed it. They saved lives. The biggest issue isn’t the test itself. It’s the definition of “significant change.” ICH Q1A(R2) says a drug has failed if its assay drops below 90% of the original value, or if impurities rise above limits, or if physical properties change. But what’s “significant”? Is a 4.8% drop in potency a failure? One regulator says yes. Another says no. A Pfizer quality analyst posted on Reddit that their team once had a submission rejected over a 4.8% drop-even though the drug was still well within therapeutic range. The regulator didn’t care about clinical relevance. They cared about the rule.What’s Changing? The Future of Stability Testing
The rules haven’t changed in 20 years. But drugs have. mRNA vaccines, antibody-drug conjugates, lipid nanoparticles-these aren’t small molecules. They’re fragile. They don’t degrade the way aspirin does. The 40°C/75% RH test doesn’t predict their failure. A 2021 FDA warning letter to Amgen cited stability issues with a monoclonal antibody that degraded during shipping. The standard test didn’t catch it. New tools are emerging. Real-time monitoring using process analytical technology (PAT) is being piloted by the FDA. Predictive modeling using high-temperature stress tests (up to 80°C) is now used by 74% of top pharma companies. Some predict that by 2030, 60% of stability data will come from models-not physical tests. But regulators are slow. The EMA rejected 8 model-based submissions in 2022 and 2023. They want data. Real data. In real time. In real conditions.What You Need to Do Right Now
If you’re developing a drug:- Don’t guess the storage condition. Pick the right ICH zone for your market.
- Run long-term testing at 25°C/60% RH or 30°C/65% RH-don’t skip it.
- Always run accelerated testing at 40°C/75% RH for 6 months-even if you think your drug is stable.
- If you’re making refrigerated products, test at 25°C/60% RH, not 40°C.
- Map your chambers. Know where the hot spots are. Calibrate monthly.
- Document everything. Every temperature reading. Every humidity log. Every failed batch.
What are the standard temperature and humidity conditions for long-term stability testing?
The two accepted conditions under ICH Q1A(R2) are 25°C ± 2°C at 60% RH ± 5% RH, or 30°C ± 2°C at 65% RH ± 5% RH. The choice depends on the climatic zone of the target market. For tropical regions, 30°C/65% RH is required. For temperate regions, 25°C/60% RH is acceptable.
How long does accelerated stability testing last?
Accelerated stability testing lasts exactly 6 months under controlled conditions of 40°C ± 2°C and 75% RH ± 5% RH. This is a global standard used by FDA, EMA, and other agencies to predict long-term degradation. The results help estimate shelf life, but they don’t replace real-time data.
Do refrigerated drugs use the same stability conditions as room-temperature drugs?
No. Refrigerated products are tested at 5°C ± 3°C for long-term storage. Their accelerated test is conducted at 25°C ± 2°C and 60% RH ± 5% RH-not 40°C. Testing at 40°C would destroy temperature-sensitive products like insulin or mRNA vaccines. The goal is to simulate accidental warming during transport or storage.
What happens if a stability test fails?
A failed stability test means the drug doesn’t meet quality standards over time. This can lead to regulatory rejection of your application, delays in approval, or even a product recall after launch. In 2022, the FDA issued 27 warning letters for stability testing failures. Companies may need to reformulate the product, extend shelf life claims, or withdraw from the market.
Are there different stability requirements for different global markets?
Yes. ICH defines five climatic zones. Products intended for Zone IVa (hot-humid tropical) must be tested at 30°C/65% RH. Those for Zone IVb (higher humidity) require 30°C/75% RH. If you’re selling globally, you must prove stability under all applicable conditions. Skipping zone-specific testing can lead to regulatory action in those countries.
Can predictive modeling replace physical stability testing?
Some companies use predictive models with high-temperature stress tests to estimate shelf life faster. However, regulators like the EMA still require real-time data for approval. While modeling is growing-used by 74% of top pharma companies-it hasn’t replaced physical testing. The FDA and EMA have rejected model-only submissions in the past. For now, physical testing remains mandatory.
How often should stability chambers be calibrated?
Chambers must be calibrated at least quarterly, and temperature mapping should be performed during qualification and after any major maintenance. The tolerance for temperature must stay within ±0.5°C and humidity within ±2% RH. A single excursion beyond ±2°C can invalidate an entire 12-month study, as shown in industry surveys where 78% of labs experienced at least one excursion.
Katie Allan
December 6, 2025 AT 07:16Stability testing is the unsung hero of pharmaceutical safety. No one cheers when a pill doesn’t turn toxic in a Singapore warehouse, but that’s exactly what this process prevents. It’s boring, meticulous work-and that’s why it works.
Regulators aren’t being arbitrary. They’re reacting to real failures: cloudy liquids, crumbling tablets, potency drops that lead to treatment failure. The 90% threshold isn’t magic-it’s the line between effective and dangerous.
I’ve seen labs cut corners on humidity control. One degree off, and your entire 12-month study becomes a paperweight. Calibration isn’t optional. It’s ethical.
And yes, the system is slow. But when you’re dealing with human lives, slow is better than wrong.
Deborah Jacobs
December 7, 2025 AT 18:55Let’s be real-this isn’t just science, it’s survival. I once worked with a team that skipped intermediate testing because ‘the drug looked fine.’ Three months after launch, patients in Brazil started reporting headaches. Turned out the tablet was absorbing moisture and leaching a byproduct. We had to recall 200k units.
That 30°C/65% RH isn’t a suggestion. It’s a lifeline for people in monsoon zones. If your drug can’t survive a Chennai summer, it shouldn’t be sold there. Period.
And don’t get me started on how some companies treat refrigerated drugs like they’re just ‘cold pills.’ Insulin isn’t aspirin. It’s a living molecule. Treat it like one.
Stephanie Bodde
December 8, 2025 AT 02:37Just wanted to say thank you for laying this out so clearly. As someone new to pharma QA, I’ve been drowning in ICH guidelines-and this feels like a flashlight in a cave.
That bit about Zone IVb at 75% RH? I’m printing that out. We’re launching in Indonesia next year and I was terrified I’d miss something.
You’re right-this isn’t glamorous. But it’s sacred work. Keep doing it.
❤️
Philip Kristy Wijaya
December 9, 2025 AT 23:09Jennifer Patrician
December 11, 2025 AT 22:59They’re lying about the 90% rule. It’s not about safety-it’s about profit. Big Pharma wants you to think the drug fails at 89.7% potency so they can force you to buy a new batch every 12 months. But the truth? That drug still works fine at 82%.
Remember the 2022 FDA warning letters? 23 of them were against companies that used non-US made chambers. Coincidence? I don’t think so.
And why do they ignore freeze-thaw cycles for mRNA? Because they don’t want you to know how fragile these ‘miracle vaccines’ really are. They’re barely held together by hope and tape.
They’re not protecting you. They’re protecting their stock price.
Mellissa Landrum
December 12, 2025 AT 11:39Mark Curry
December 13, 2025 AT 12:32It’s quiet work. No applause. No headlines. Just a lab tech checking humidity logs at 2 a.m.
But that’s the moment when someone’s life gets saved. Not because of a breakthrough drug-but because the pill they took didn’t turn into poison.
I’ve been in this field 18 years. Seen too many shortcuts. Seen too many recalls.
Do the right thing. Even when no one’s watching.
🙏
aditya dixit
December 15, 2025 AT 03:15As someone from India, I’ve seen firsthand what happens when stability testing is ignored. A generic insulin batch I used in 2019 lost potency in monsoon humidity. My HbA1c spiked. I almost ended up in the hospital.
The 30°C/65% RH condition isn’t a formality-it’s survival. We don’t have climate-controlled homes. Our pharmacies are open-air stalls. If your drug can’t handle that, you shouldn’t be selling it here.
And yes, calibration matters. I’ve seen chambers with 3°C drift. That’s not a glitch. That’s negligence.
Thank you for writing this. People in the Global South need to hear it.
Mark Ziegenbein
December 17, 2025 AT 01:00One must confront the profound epistemological crisis at the heart of contemporary pharmaceutical regulation: the conflation of empirical data with moral authority. The ICH Q1A(R2) framework, while ostensibly grounded in empirical observation, functions as a quasi-religious dogma-a liturgical script recited by bureaucrats who mistake procedural fidelity for scientific truth.
The 40°C/75% RH accelerated condition is not a predictive model-it is a performative ritual. It was designed for aspirin, not lipid nanoparticles. To insist on its universal application is to commit the fallacy of reification-to treat a metaphor as a mechanism.
And yet, the EMA’s rejection of model-based submissions reveals not conservatism but epistemic cowardice. They fear the uncertainty of prediction. They crave the illusion of control. They would rather drown in 12 months of physical testing than risk the ambiguity of a predictive algorithm.
What is stability, truly? Is it a number? Or is it a narrative we construct to soothe our existential dread of decay?
Perhaps the most stable thing in this entire system… is the silence of those who know it’s all a charade.